BiQ: Urogen @ the TD Cowen Oncology Innovation Summit 2025 (URGN)
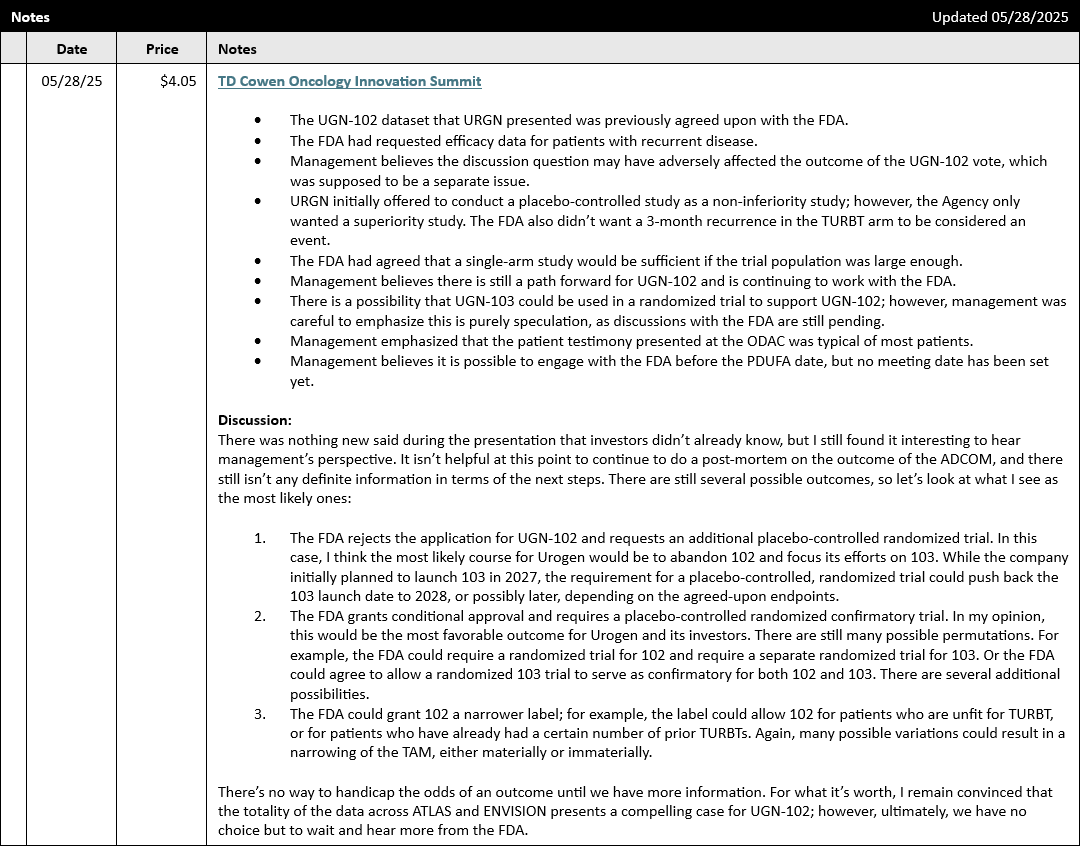
There was nothing new said during the presentation that investors didn’t already know, but I still found it interesting to hear management’s perspective after the unfortunate outcome of the ADCOM. It isn’t helpful at this point to continue to do a post-mortem on the result of the vote, and there still isn’t any definite information in terms of the next steps. There are still several possible outcomes, so let’s look at what I see as the most likely ones:
- The FDA rejects the application for UGN-102 and requests an additional placebo-controlled randomized trial. In this case, I think the most likely course for Urogen would be to abandon 102 and focus its efforts on 103. While the company initially planned to launch 103 in 2027, the requirement for a placebo-controlled, randomized trial could push back the 103 launch date to 2028, or possibly later, depending on the agreed-upon endpoints.
- The FDA grants conditional approval and requires a placebo-controlled randomized confirmatory trial. In my opinion, this would be the most favorable outcome for Urogen and its investors. There are still many possible permutations. For example, the FDA could require a randomized trial for 102 and require a separate randomized trial for 103. Or the FDA could agree to allow a randomized 103 trial to serve as confirmatory for both 102 and 103. There are several additional possibilities.
- The FDA could grant 102 a narrower label; for example, the label could allow 102 for patients who are unfit for TURBT, or for patients who have already had a certain number of prior TURBTs. Again, many possible variations could result in a narrowing of the TAM, either materially or immaterially.
Of course, this is not an exhaustive list of possible outcomes, and there’s no way to handicap the odds of a specific outcome until we have more information. For what it’s worth, however, I still believe that the totality of the data across ATLAS and ENVISION presents a compelling case for UGN-102, though the odds of a clean approval have dropped significantly after the results of the ADCOM (to put it mildly). Ultimately, we have no choice but to wait until we hear more from the FDA.
Please refer to the BiQAP Live spreadsheet on the Active Portfolio page or the iQCS for additional information.
URGN share price at time of publication: $4.05
Not a BiQ Premium member, or have a friend who may be interested? Try BiQ Premium for $10 (80% off) for the first month, or $250 (50% off) for the first year.
Biotech iQ is 100% subscriber supported. If you find this information helpful, please spread the word. You can also follow me on X @ Biotech iQ (_Biotech_iQ).
Biotech iQ is not an investment professional, and nothing on this page or this website should be considered investment advice. Please consult with a licensed investment professional as necessary. Past performance is not indicative of future results.
Member discussion