BiQ: Recap for the Week Ending April 18, 2025
Welcome to the BiQ Weekly Recap #13!
I want to welcome the new Biotech iQ members who joined this past week--thank you for joining the BiQ Community. I've been a biotech investor for over two decades, but BiQ is a new service, and I'm humbled and grateful for its warm reception from the biotech community.
If you haven't already done so, I encourage members to join the BiQ Community Chat Server on Discord.
Even if you don't have time to participate regularly, BiQ Chat is still the quickest way to receive updates, trade alerts (Premium only), and answers to questions.
Premium members have access to the Public and Premium channels, while Public members can access the Public channels. Click here for instructions on how to access the BiQ Community Chat Server.
While I publish several articles and updates each week, please remember that Biotech iQ is much more than a newsletter service. BiQ's most valuable tools are found on the Biotech iQ website (www.biotechiq.com). These include:
- The Active Portfolio displays a list of all companies currently included in Active Coverage, together with their outlook and ratings.
- The Catalyst Tracker & Events Calendar displays a quarter-by-quarter list of upcoming catalysts and a calendar of forthcoming events.
- The News Feed displays an RSS feed of all press releases from companies covered at Biotech iQ (if the company provides RSS services).
- BiQ Community Chat
Weekly Overview
The XBI closed last Friday at 74.30, opened Monday at 71.60, and closed Friday at 75.95. It reached an intra-week high of 77.56 and a low of 71.07, an intra-week range of just over 9%.
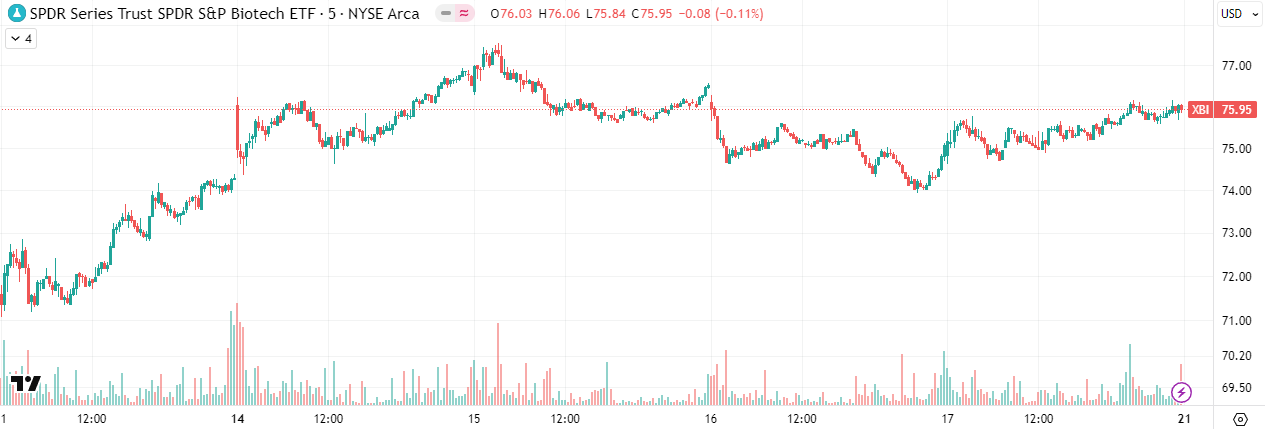
Zooming out to the one-month chart, we can see the index seemed to bounce off the 66 level. It's difficult to determine whether this remains a short-term technical bounce or the beginning stages of a recovery. Until proven otherwise, I plan to remain cautious and to expect continued volatility.
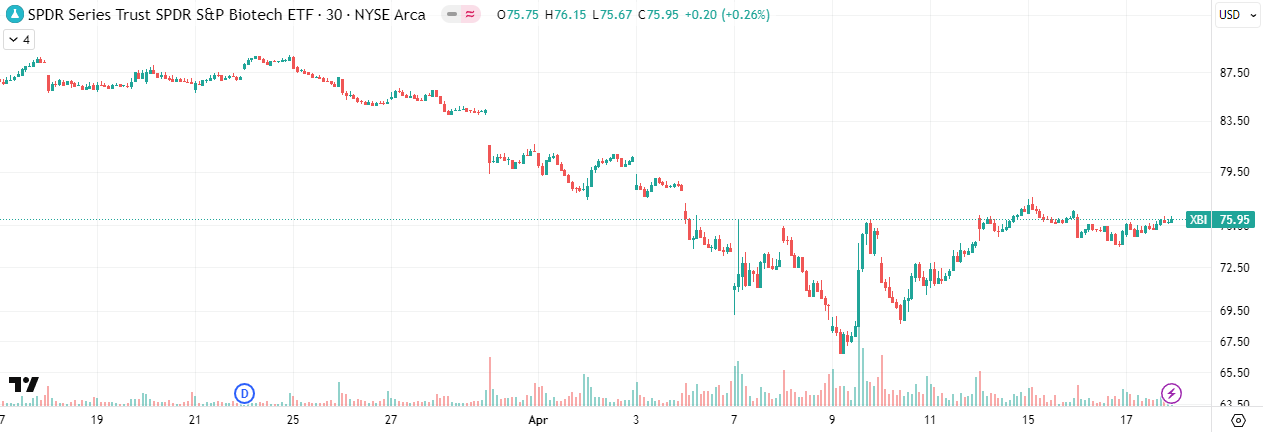
Zooming out further to the five-year chart, we can see that the index bounced on high volume from the 66-ish level, almost reaching 5-year lows near 64. The RSI remains oversold. From this perspective, I feel cautiously optimistic that the worst damage might be behind us; however, as I mentioned above, I prefer to remain cautious and hold excess liquidity until we see a confirmed uptrend.
In times of high volatility, it's more important than ever to remember a few fundamental principles:
- The US biotech industry remains as innovative and dynamic as ever, only at much more attractive valuations than we've seen for a while.
- Despite disruptions at the FDA, I believe the agency remains committed to fostering innovation–though there may be some bumps along the way. The US biotech industry remains the envy of the world, and it's in no one's interest to change that.
- Over the long term, companies will continue to trade on fundamentals. Buying quality stocks when they're cheap and selling when they're expensive is still a winning strategy for long-term investors.
That said, proper risk management is critical to long-term success and to avoid the permanent destruction of capital, especially in times of increased market volatility. This means maintaining adequate liquidity reserves to remain rational and focused while others panic.
While I expect volatility may be the new normal for the foreseeable future, I am cautiously optimistic that market conditions will soon begin to stabilize; however, I still plan to maintain a more defensive posture until there are clear signs of a trend reversal. We're not there yet, but I think we're getting closer.
Industry News
Last week, Megyn Kelly's interview with Dr. Marty Makary was posted on Youtube. Here's the interview for those who might have missed it.
Since most of the communication staff at the FDA has been terminated, this was one of the first opportunities for investors to get an early sense of the FDA's future direction under Makary. Putting aside the politicizing that ran throughout the interview, here are some of the important highlights that stood out to me:
- Streamline bureaucracy and redundancy throughout the agency by integrating departments that currently operate as silos, improving information sharing, and implementing improved technology solutions.
- Streamline drug approval processes, especially for ultra-rare diseases, with greater emphasis on post-approval monitoring to reduce the burden of multiple randomized trials.
- Reduce reliance on animal testing by leveraging technology and computer models.
- Emphasize the role of food and nutrition.
- Reduce the pharmaceutical industry's influence throughout the Agency (one example is by removing industry members from advisory committees.)
- Assurances that recent staffing cuts would not adversely impact timelines for application reviews and drug approvals.
I am hopeful that the interview might help to calm investors' nerves, which have been understandably shaken by the recent news flow and rapid changes at the Agency. I think the goals laid out by Makary are sensible; however, I think actions are more important than words. I believe that the FDA will remain committed to innovation over the long term; however, in the near term, I plan to maintain a healthy level of caution until we have more concrete evidence that everything remains on track at the Agency.
In other news, President Trump issued a new Executive Order last week directing HHS and Congress to find ways to:
- Reduce drug costs.
- Reduce the influence of middlemen (such as PBMs).
- Expand drug pricing negotiations.
- Streamline drug development.
- Improve access to generics and biosimilars.
- Remove the "pill penalty", which currently penalizes the development of small-molecule oral drugs.
- Streamline approval of cross-border pharmaceutical purchasing.
Since the EO only laid out high-level directives as opposed to actual policy proposals, it's impossible to accurately predict the eventual impact of these directives at this early stage. Several initiatives here could eventually be positive for the industry, although there will undoubtedly be some winners and losers. As always, the devil is in the details, and we don't have any details yet. Nothing in the EO changes my outlook for any of the names in the BiQAP; however, I plan to remain vigilant for further news and developments.
BiQ Service Updates
- A new format for the BiQAP spreadsheet has been published. The following features have been added:
1. Strong Buy and Buy Targets
2. Price Quotes (delayed up to 15 minutes)
3. iQ Scores
4. Market Cap and EV
5. Valuation Ratios (EV and Adjusted EV Ratios)
6. Forward CAGR Estimates
7. Forward Revenue Estimates
Members can now view a live version of the BiQAP, which is updated in real-time, and a snapshot image, which is updated once weekly. Please visit the Active Portfolio page for details.
BiQ Membership Discounts
For free members interested in an annual Premium membership to Biotech iQ, I am now offering two new discount options for a limited time.
50% Off your First year: https://www.biotechiq.net/50-off-your-first-year
20% Off for Life: https://www.biotechiq.net/20-off-for-life
Click here for more information about Biotech iQ, and to see what's included in a Premium membership. A free 7-day trial and monthly membership options are also available at www.biotechiq.net.