Biotech iQ Recap for the Week Ending May 23, 2025
Welcome to the BiQ Weekly Recap #18!
If you are a new BiQ Free or Premium subscriber, remember to join the BiQ Chat server on Discord. Click here for more information. Also, please read the Getting Started article for more information on the tools and services offered at BiQ.
Weekly Overview
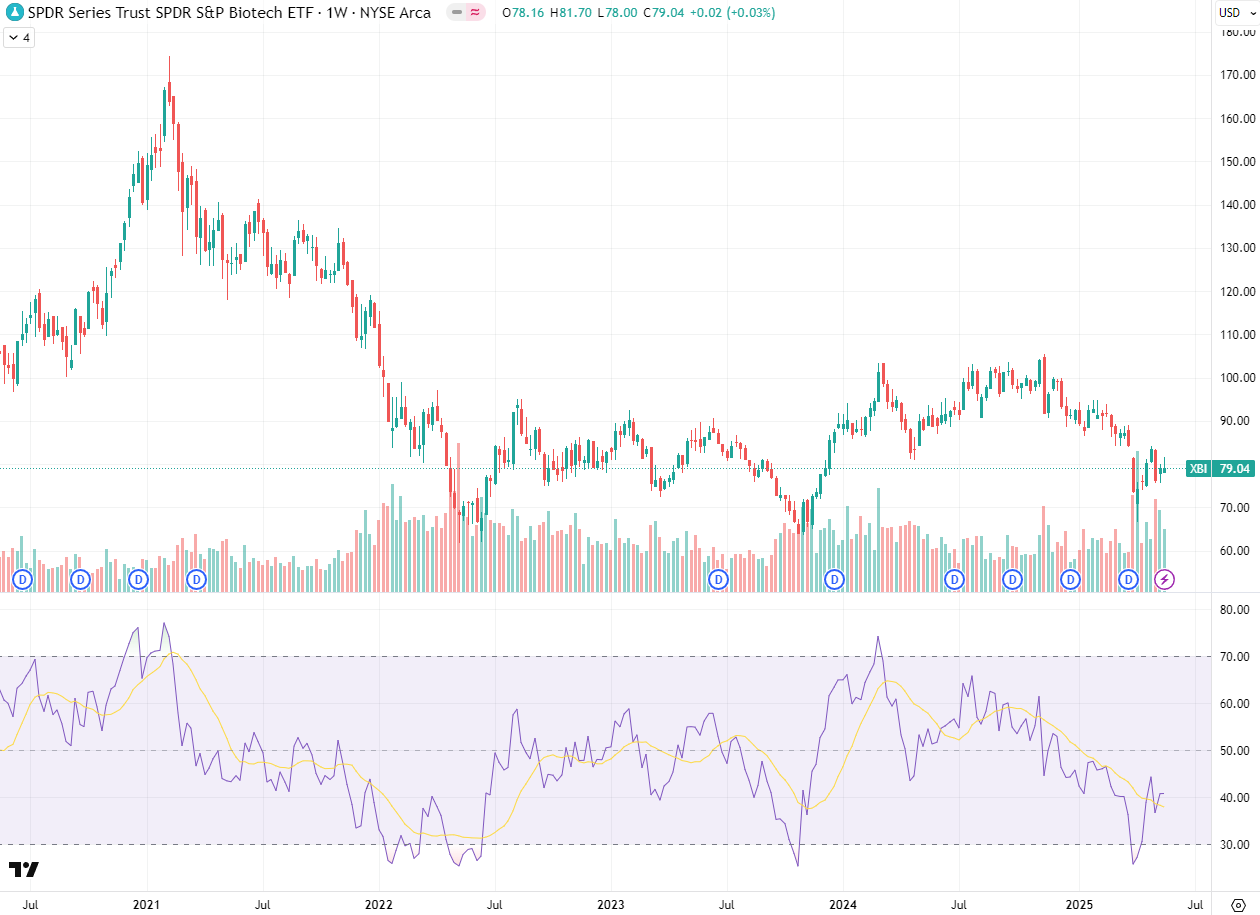
The index closed last Friday at 79.01, opened Monday at 78.32, and closed Friday at 79.06 for a weekly gain of 0.0%. It reached an intra-week high of 81.70 and a low of 78.14 for an intra-week range of 4.6%.
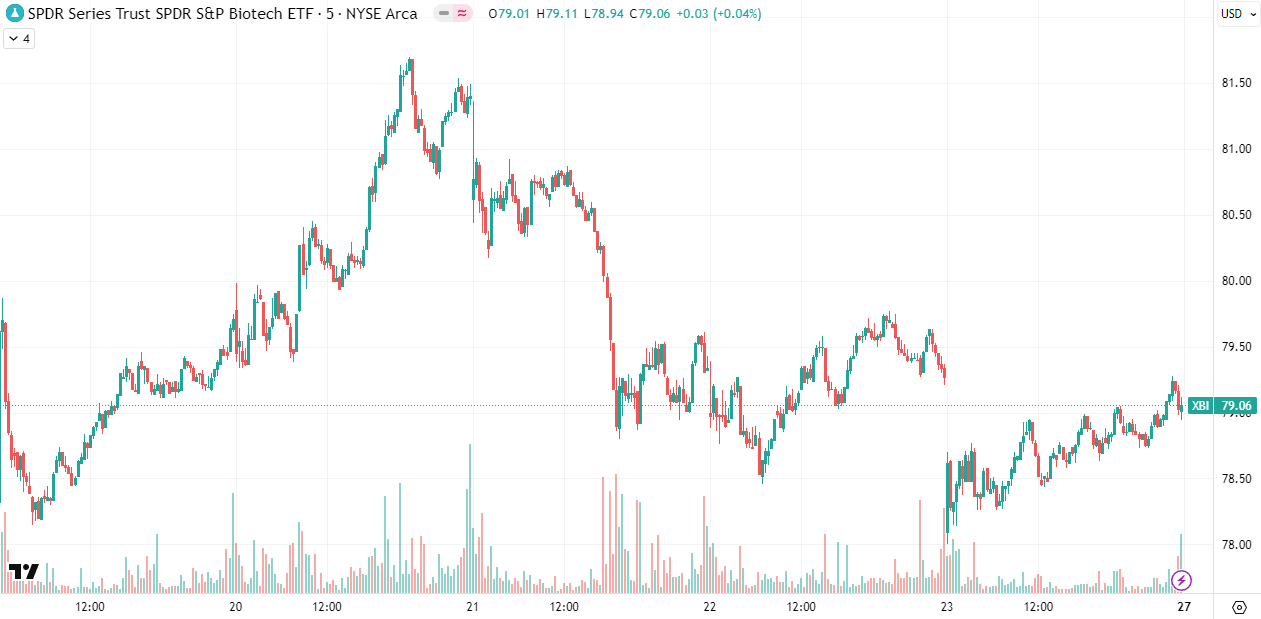
Zooming out to the one-month chart, we can see that following the strong run in April, the index still seems to be struggling to find direction, and volume remains muted. I believe markets still face significant headwinds from ongoing trade negotiations and uncertainty regarding the future direction of the FDA. I expect we can add anxiety over the implications of MFN (Most Favored Nation) pricing to the list as well. We still have a long way to go before we're clear of these headwinds, and it will take time to work through these uncertainties. I continue to maintain elevated liquidity levels and aggressively sell Call options while waiting for the index to find its footing.
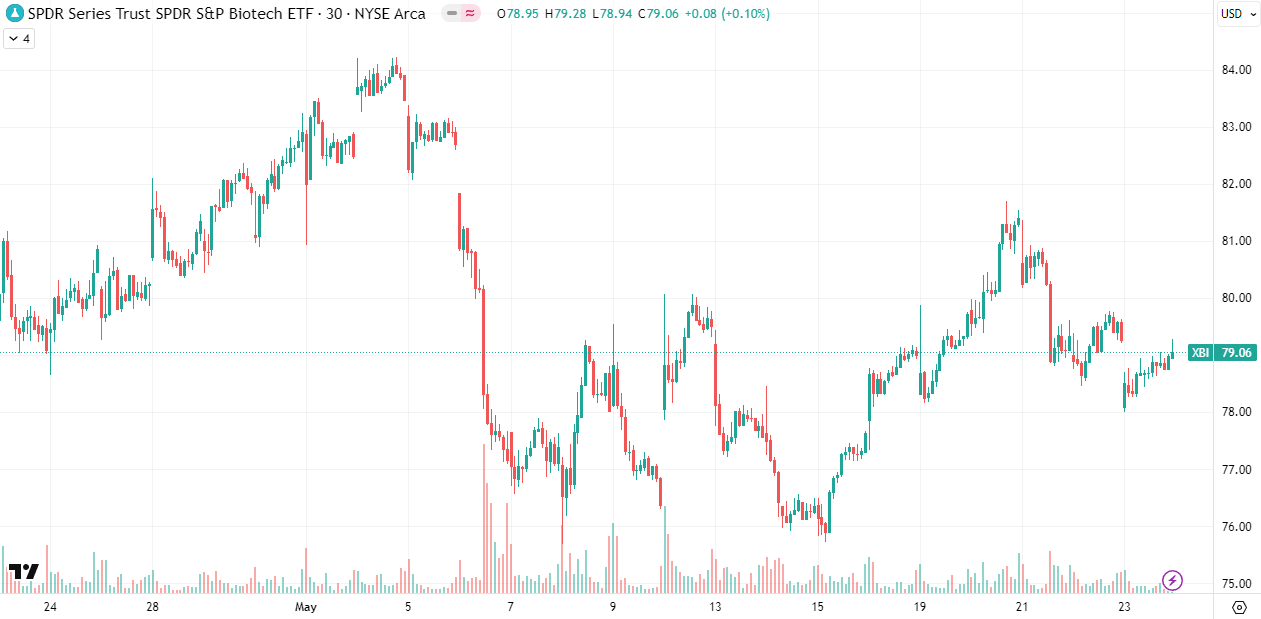
Zooming out further to the five-year chart, we can see that the index has bounced off the 64 to 66 level three times: in June 2022, October 2023, and most recently in April 2025. While I am cautiously hopeful that we may see a recovery to the 88-90 resistance level, volatility remains elevated, and it's equally possible the index revisits recent lows. It's tough to make any predictions, so my portfolio positioning remains cautious.
Biotech Industry News & Commentary
An interesting article published this past week in BioSpace summarizes the state of current checkpoint inhibitors (see Deep Dive: Checkpoint Inhibitors at a Crossroads).
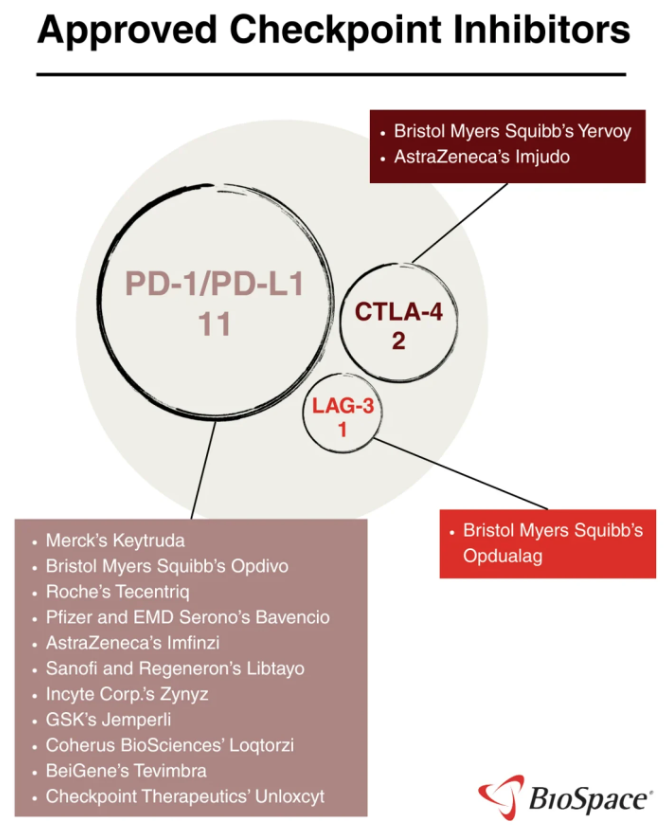
By now, most of us are familiar with existing PD-1/PD-L1 and CTLA-4 inhibitors. BMS currently has the only approved LAG-3 inhibitor, although IMMP is quickly advancing its Novartis-partnered LAG525. Recently, however, most of the attention seems to be on PD-1xVEGF inhibitors, a race that has quickly become very crowded, especially with Pfizer's recent in-licensing of SSGJ-707 from the Chinese biotech company, 3SBio, in an eye-opening deal worth up to $6 billion bio-bucks.
One area, however, that doesn't seem to get much attention is the development of oral checkpoint inhibitors by companies including, among others, privately held OmRx, BeiGene, and Arbutus. Small-molecule IO drugs could offer several advantages over existing therapies, including more convenient administration, enhanced tissue penetration, reduced immune-related adverse events, and lower healthcare costs. While it's too early to draw any conclusions, I think it's an interesting area of ongoing development that doesn't currently get much attention.
In other IO-related news, Halozyme recently filed its much-anticipated lawsuit against Merck for Merck's development of a subcutaneous formulation of Keytruda. To review, Merck licensed a hyaluronidase called ATL-B4 from South Korean company Alteogen to develop its subcutaneous formulation; however, Halozyme alleges that Merck's SC Keytruda infringes on 15 of its patents.
I'm not a patent attorney, so I won't try to anticipate the outcome of this action. However, I always knew this day would come. The development of subcutaneous formulations of large molecule drugs can only accelerate, and nobody wants to feel that their path to market relies on a single company.
Perhaps Merck and Halozyme will come to an amicable, or at least workable, settlement. What I find interesting, however, is what the outcome of this lawsuit could mean for the industry. Halozyme wants to be the sole option for companies seeking to produce subcutaneous formulations of large-molecule drugs. Merck's challenge to Halozyme's hegemony could represent a long-term existential threat to Halozyme, making the outcome of the lawsuit particularly critical for the company. On the other hand, should Halozyme prevail, it could potentially create a near-monopoly for Halozyme around the use of hyaluronidases. I have no doubt that Merck licensed ATL-B4 with full knowledge that Halozyme would eventually file a lawsuit, and I expect both sides are well prepared for the fight. I will be watching further developments with keen interest.
Who knows, the outcome could make oral formulations of immune checkpoint inhibitors much more interesting.
BiQ Service Updates
- No BiQ service updates.
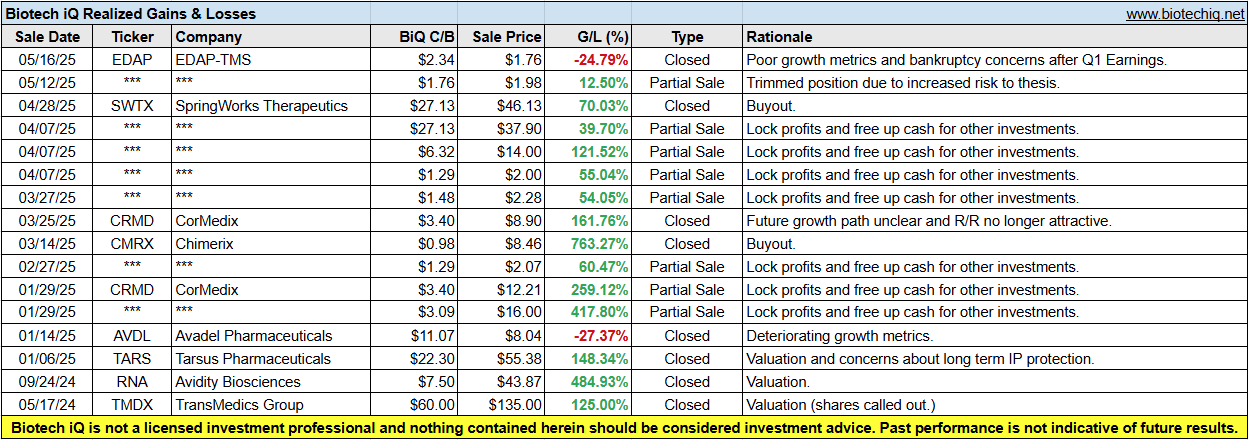
BiQ Realized Gains & Losses
Biotech iQ is oriented towards long-term investing; it is not an active trading service. Typically, I only fully exit positions if the long-term thesis and risk/reward no longer appear attractive. The BiQ Realized Gains & Losses spreadsheet displays all realized gains and losses since BiQ was launched last year. Premium members can also view the current performance of the BiQ Active Portfolio on the Active Portfolio page of the Biotech iQ website.
BiQ Premium Membership Promotions
For subscribers interested in a Premium membership to Biotech iQ, I am now offering three promotions for new subscribers:
- 50% Off your First year: https://www.biotechiq.net/50-off-your-first-year
- 20% Off for Life: https://www.biotechiq.net/20-off-for-life
- Try 1 Month of BiQ Premium for $10: https://www.biotechiq.net/first-month-for-10
Click here for more information about Biotech iQ, and to see what's included in a Premium membership, or please visit www.biotechiq.net. Promotional offers can be used only once, and only one promotional offer can be used per subscriber.
Thank you for subscribing to Biotech iQ and reading this Weekly Recap. Premium Members can continue reading below.